What are Acids and Bases? How do they act in daily life?
Acids and bases are our main courses during this journey of the class. We have read the article in CK12 to understand more about them. They look definitely similar but somehow they are different. Then, what are they?
Acid
Therefore, what is an acid, so acid is an ionic compound that creates positive hydrogen (H+) when dissolved in water. Sour taste is the property of acid, which all sour tastes are acid such as lemon, orange and more (warning: Never taste an unknown substance ). Moreover, the acid also conducts electricity because it consists of charged particles in the solution. Acid is also important in the world because some products contain acid. For example, sulfuric acid is used to manufacture many products such are paper, paint, and detergent. There is also a way to detect acid, which we use an indicator. An indicator will change the color when acids come to contact with. An example of the indicator is a compound named red cabbage. Red cabbage is a liquid chemical that will changes the color depends on how much acid contain in some products including vinegar, sodium bicarbonate, bleach and more. You can also try to test the acid in your drinks too like coca cola, or sprite. While pouring the red cabbage indicator in another liquid, combine together will change the color. Then you can use the pH scale to notice whether how high or low is the acid in that liquid.
Base
A base is an ionic compound that produces negative hydrogen (OH-) when dissolved in water. Also base shares its own certain property as well, including bitter taste. All bases have bitter taste including soap, coffee and more. In addition, the base feels slippery, you can imagine how slippery soap is. Therefore, soap is also including in base. Like acids, bases also conduct electricity because they consist of charged particles in the solution. Bases incorporate in certain products including soap, bleach, and so on. We can use red cabbage indicator to detect both acids and bases.
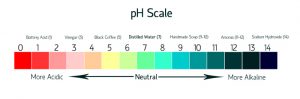
This picture shows the level of pH Scale. Under the pH of 7 are acids and above the pH of 7 are bases.