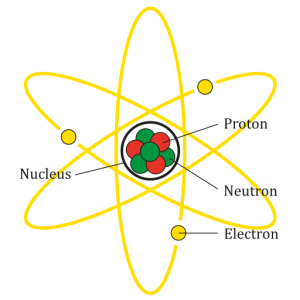
In the round 1 of Stem class, we learned about matter and atom. Matter is anything that has mass and occupied space. Atom is the smallest particle in chemical that can exist. In atom there are nucleus and electrons. And in Nucleus, there are protons and neutrons. Protons have the positive charge. Electrons have the negative charge. Neutrons have the zero charge. Even neutrons have zero charge but the number of neutrons are able to create more mas because they mass as about protons and electrons together. All the elements should have the same amount number of electrons and protons. it’s called neutral atom is when the electrons and protons are balance. Ion is when the atom losing or gain more electrons. It means that the electrons and protons are not balance. Ion has 2 types:
1. Cation is when the atoms have more protons than electrons.
2. Anion is when the atoms have more electrons than protons. Again the protons didn’t lose or gain more only the electrons that loose and gain.
Now let’s move to isotope. Isotope has the same amount of protons and electrons but doesn’t have the same amount of neutrons. Atomic number is the total number of protons. Atomic mass is the total mass of an atom.
The picture is from Wikipedia.