Chemical Bonding
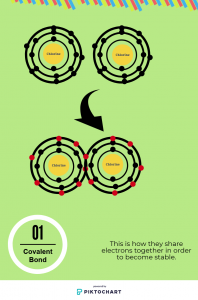
Covalent bond is the bonding between nonmetal and nonmetal that share the valence electrons in order to be complete or stable. When the elements are stable, they won’t share electrons again because they already completed. To add on if they are completed they will have eight valence electrons and inner electrons are two. For instance, there are two elements, chlorine and chlorine. They are nonmetal groups. They each have 2 electrons inner shelf and have 7 electrons outer shelf. That’s mean they need 1 more to complete. Two of them can share one of their electrons together so each of them will have eight because they each share one electrons. Now they are complete so they won’t react with other elements. To summary the instance covalent bond can share more than one electrons in order to become stable.
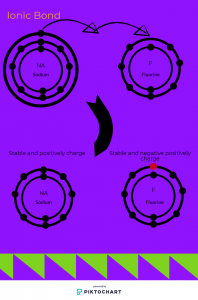
Ionic bond is the bonding between nonmetal and metal. In this type of bond they don’t share means that they gain or lose valence electrons. Usually metallic gives out valence electrons to nonmetallic to become positively charge and nonmetallic that gets the electrons from metallic is negatively charge.( Example is below)